

| Title | Nationality | Name | Gender and age | First Date Elected | Date Elected | Term (Years) | Education |
|---|---|---|---|---|---|---|---|
| Chairman | R.O.C | Wei-Chyun Wong | M | 2001.12.17 | 2022.06.21 | 3 | PHD in Chemistry, University of Pennsylvania |
| Director | R.O.C | Shiang-Li Chen | M | 2001.12.17 | 2022.06.21 | 3 | MBA, University of Georgetown |
| Director | R.O.C | Mercuries & Associates Holding, Ltd. Institutional representative : Chin-Hsin Hsu | F | 2001.12.17 | 2022.06.21 | 3 | Master of Laws, Northwestern University, USA Aaaistant partner lawyer of Wanguo Law Firm Judge of Keelung District Court, Taiwan |
| Director | R.O.C | Mercuries & Associates Holding, Ltd. Institutional representative : Wen-Chih Chou | M | 2001.12.17 | 2022.06.21 | 3 | PHD of Chemistry, National Taiwan University |
| Independent Director | R.O.C | Te-cheng Tu | M | 2013.06.18 | 2022.06.21 | 3 | MBA, University of Houston |
| Independent Director | R.O.C | Chia-Chun Jay Chen | M | 2015.06.12 | 2022.06.21 | 3 | PHD of Chemistry, Unviversity of Harvard |
| Independent Director | R.O.C | Vincent Wang | M | 2022.06.12 | 2022.06.21 | 3 | Double Major Master's Degree of Finance and Entrepreneurship Management, Wharton School of the University of Pennsylvania Director, EASYCARD Corp. and TAIWAN SUGAR Corp. Director of TVCA |
| Title | Nationality | Name | Gender | Date Effective | Major experience / Education | Currently Ohter Position |
|---|---|---|---|---|---|---|
| President | R.O.C | Wen-Chih Chou | M | 2002.06.01 | PHD in Chemistry, National Taiwan University | Director of Yushan Pharmacenticals Inc. Biosciences, Inc.. |
| Business Div. Vice President | R.O.C | Michele Seah | F | 1998.12.01 | BS in Agricultural Chemistry, National Taiwan University | None |
| Technical Div. | R.O.C | Jinun Ben Yeh | M | 2007.07.01 | MS in Chemistry, National Tsing Hua University | None |
| F&A Div. Vice President / Spokesman / CG Officer | R.O.C | Deiter Yang | M | 2003.01.01 | MS in Accounting, Tamkang University Certified Public Accountant Mercuries & Associates Holding, Ltd. Finance Manager SCI Pharmtech, Inc., F&A Manager | Supervisor of Yushan Pharmaceuticals Inc., Framosa Co., Ltd., HoneyBear Biosciences, Inc.. |
| Operating Div. Assistant Vice President | R.O.C | Wei-Song Yin | M | 2015.07.01 | MS in Chemistry, National Tsing Hua University ITRI Researcher SCI Pharmtech, Inc., R&D Manager | None |
| Quality Div. Assistant Vice President | R.O.C | Bo-Fong Chen | M | 2017.10.13 | Ph. D in Chemistry, National Sun Yat-Sen University SCI Pharmtech Inc., QC Manager | None |
| PDM&EV Manager | R.O.C | Ricky Liu | M | 2002.01.01 | MS in Chemical Engineering, National Tsing Hua University SCI Pharmtech, Inc., PD Manager | None |
| QA &RA Manager | R.O.C | Vincent Chiang | M | 2002.12.16 | MS in Chemistry, National Cheng Kung University | None |
| BA Manager | R.O.C | Nancy Lee | F | 2009.05.01 | MS in Chemical Engineering, National Tsing Hua University | None |
| EN Manager | R.O.C | Chung-Lung Su | M | 2022.03.01 | MS in Chemical Engineering, National Cheng Kung University SCI Pharmtech, Inc., EN Director | None |
| R&D Deputy Manager | R.O.C | Andy Lee | M | 2023.07.05 | MS in Chemistry, National Cheng Kung University Daxin Materials EN Deputy Manager AUO Deputy Manager SCI Pharmtech Inc., R&D Chief Commissioner | None |
| PD Deputy Manager | R.O.C | Jimmy Chang | M | 2023.07.05 | BS of Chemical Fiber Department, National Taiwan Institute of Technology Hualong Company Section Manager SCI Pharmtech, Inc., PD Account Manager | None |
| QC Deputy Manager | R.O.C | JoJo Lu | F | 2023.07.05 | MS in Chemistry, York University Yaohua Biotechnology Research specialist Formosa Laboratories, Inc. Associate Researcher SCI Pharmtech Inc., QC Deputy Chief Commissioner | None |
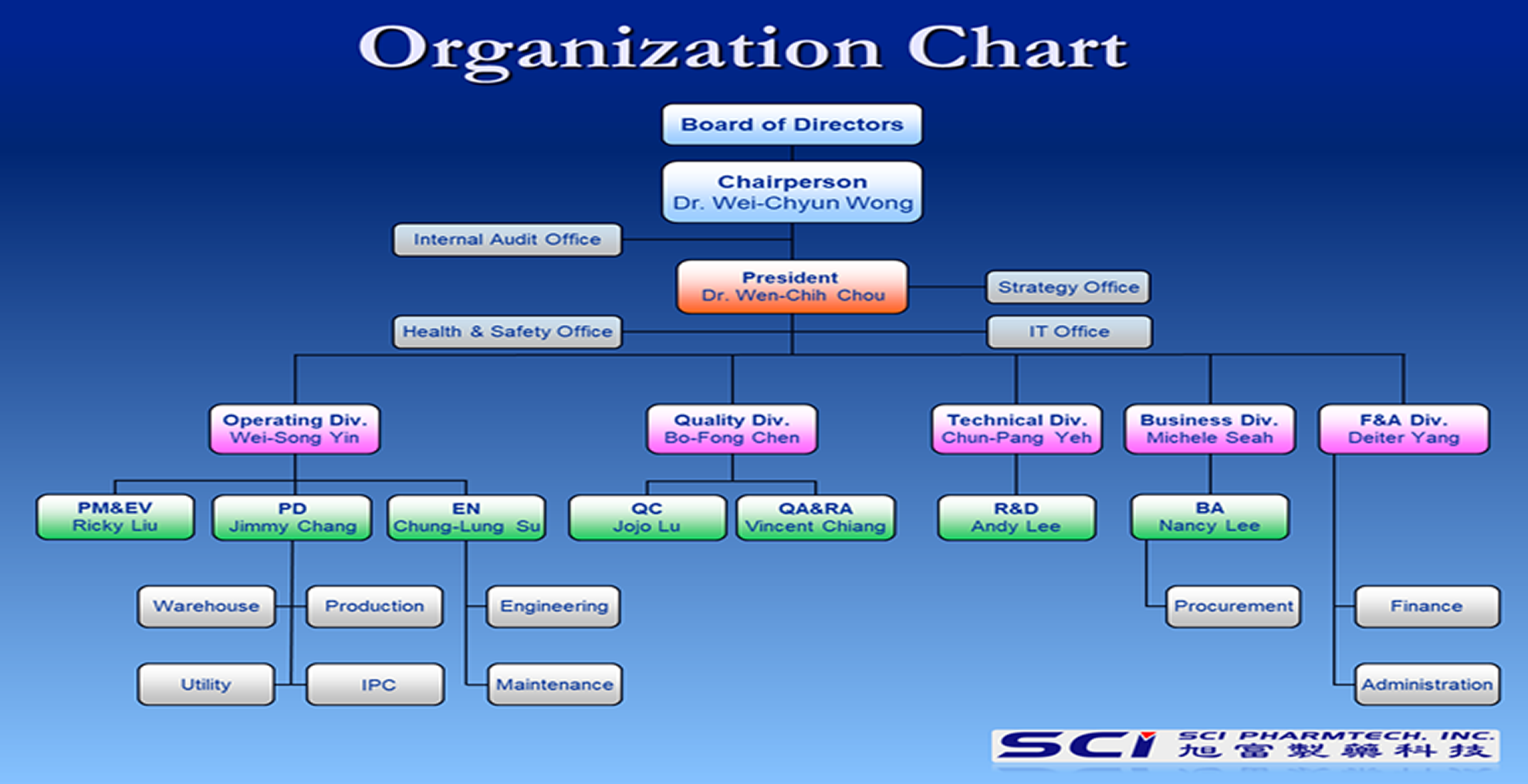
Introduction to Organizational Functions
| President | Market Trends and strategy planning. Setting up company goals and operational directions. Establishing organizational strategy and goals of different units. Executing the improvement plan of performance. Decision-making and management. |
| Audit Office | Executing the annual audit plan. Establishing, amending, and executing the internal control system. Establishing, amending, and executing the internal audit implementation rules. |
| Strategy Office | Grasp market trends Planning organizational strategy |
| IT Office | In charge of planning the structure of the Information System and the management of the related equipment. Purchasing, Installing, and maintaining information equipment, including software and hardware. Maintenance of the Internet structure. |
| Safety Office | Establishing, maintaining, and supervising industrial safety. Promoting and maintaining OHSAS18001 Management Systems. |
| Operating Div. | Operating Div. In charge of integrating the management and supervision of the Production Department, Production Management Department, Environmental Protection Department, and Engineering Department. Promoting and maintaining GMP, ISO 9001, ISO14001, OHSAS18001 Management Systems. Tacking the GMP and environmental safety audit affairs with the nationwide and overseas administrative agencies and customers. Production capacity planning and construction. |
| PD Establishing and enforcing production schedules, monitoring subordinate units to ensure smooth production, and troubleshooting incidents to fulfill all operation goals. Adequately integrate and coordinate the correspondence of the production department and other units. Arranging the operations to realize the production plan. | |
| EN Planning and executing engineering plans; bargaining, purchasing, out-sourcing of equipment, while coordinating with other units. For equipment, computer software, and hardware engineering projects: Enacting the regulations, bargaining, out-sourcing, supervision, and acceptance. Ensuring the equipment’s compliance with cGMP. Verifying the qualification of the outsourcer’s ability. Arranging, updating, and executing the maintenance and repair plan. Maintenance and repair of machinery equipment. Bargaining and supervising the outsourced equipment repairs. | |
| PM&EH Adjusting the production and marketing schedule, as well as establishing the production order. Thoroughly acknowledging the quantities of raw materials and final products. Estimating the shipments with the production and marketing schedule, as well as enforcing production progression regularly and coordinating the shipment schedule accordingly. Planning and executing the safety classification, labeling an0d packaging system of chemicals. Enhancing and maintaining the ISO14001 Management System, implementing related practices in air pollution, water pollution, waste, and toxic chemicals regularly. Planning on the operational practice and equipment for air pollution, water pollution, soil pollution, waste, toxic chemical, and noises, and assisting in solving abnormal issues in operational practice. Planning on the record and declaration practices for air pollution, water pollution, waste, and toxic chemicals, while also coordinating and integrating the internal declaration practice, confirming the declaration content, and corresponding to the consequent external audits. | |
| Business Div. | Establishing the goals of marketing to ensure the market share and profitability of the products. Understanding the current and future needs and expectations of the clients, including the quality compliance, availability, distribution, customer service, pricing, responsibilities of the products, impacts on the environment, etc. Acknowledging the purchasing requirement and specifications timely and effectively. Evaluating the vendors and understanding the trend of market prices. |
| Technical Div. | R&D Enacting the development and progress of the new products projects. In charge of providing personnel executing the plans, whose task shall include the evaluation, discussion, and training for every research project. Providing the necessary technical support with production operations. Providing the evaluation report on the client commission or the collaboration projects. |
| Quality Div. | QC In charge of the training and management of the quality control personnel. Enacting and amending the evaluation methods and producing evaluation reports. Confirming the calibration of analytical instruments, SOP, and the validation of the analytical methods. Confirming the quality control and sampling personnel’s operations and reports. |
| QA&RA Establishing a quality management system, request all of the personnel to comply and ensure execution. Maintaining the quality system and introducing related laws and regulations from foreign nations. Reviewing all documents related to quality thoroughly. Introducing new laws and regulations timely and maintaining the effectiveness of the existing control registration to ensure the quality system meets the requirements of cGMP. Leading and receiving quality control from organizations of laws and regulations or clients domestically and internationally, as well as performing internal audits and regular suppliers’audits. | |
| F&A Div. | Finance In charge of accounting, taxation, budgeting, etc, to provide financial information as references for managerial decision-making. In charge of fund procurement and corresponding with banks. Operating and processing the business of bonded factories. |
| Administration Managing and planning human resources, establishing and executing the personnel system. Tackling daily administrative affairs and other operations, such as maintaining the tidiness of the plant. |

