- RD_01_mRD_01_mSCi pharmtech, Inc., has been passed GMP audit by worldwide authorities from USFDA, EDQM, PMDA, KFDA, TFDA since 2003
- We have a strong and dedicated tech. teams who is responsible for process development optimization, scaling up, and analysis method development/validation.
- The project management team is focus on communicating with customers and quickly responding to customer needs, we are the best communication bridge for clients.
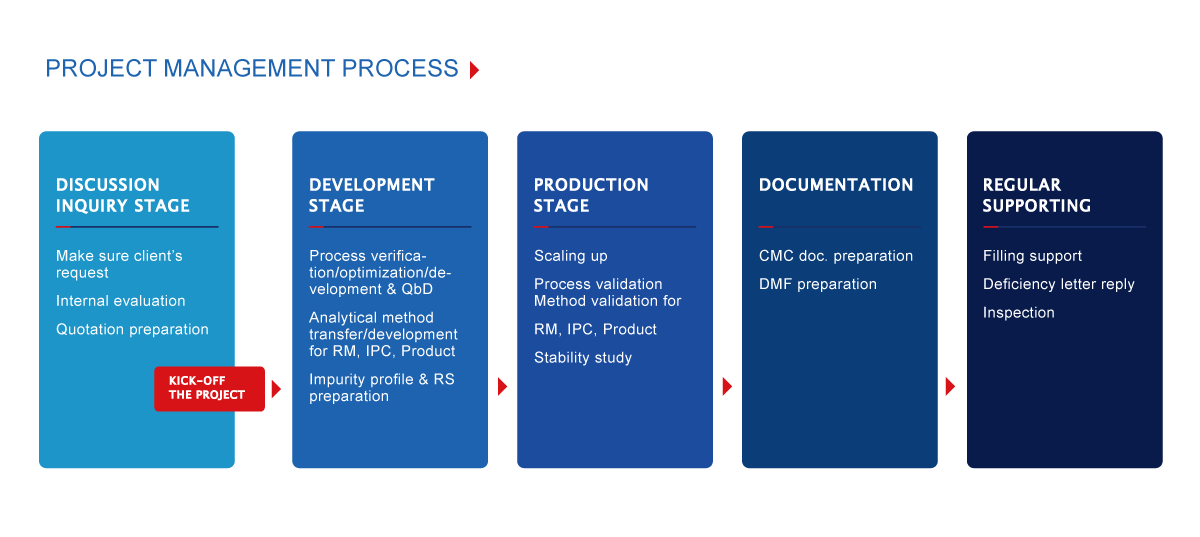
- The flow of project evaluation and execution is as follows:
SOP in project management